|
 |
Chemical properties of Radium - Health effects of Radium - Environmental effects of Radium
|
 |
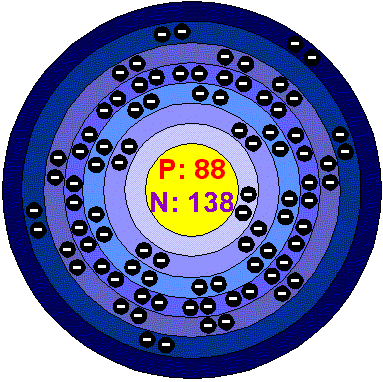
RadiumRadium is silvery, lustrous, soft, intensely radioactive. It readily oxidizes on exposure to air, turning from almost pure white to black. Radium is luminescent, corrodes in water to form Radium hydroxide. Although is the heaviest member of the alkaline-earth group it is the most volatile. Applications Radium is used in luminous paint (in the form of Radium bromide). Radium and Beryllium were once used as a portable source of neutrons. Radium is used in medicine to produce Radon gas, used for cancer treatment. At the beginning of the 19th century Radium was used as additive in products like toothpaste, hair creams and even food items. Radium in the environment It has been estimated that each square kilometer of the earth surface (to a depth of 40 cm) contains 1 gram of Radium. Early in the twentieth century Radium was extracted from Uranium ores for use in luminous dials and medical treatment. The amount of Radium in Uranium ores varies between 150 and 350 mg/ton. The most in contained in the ores of Zaire and Canada. Health effects of RadiumRadium is naturally present in the
envIronment in very small amounts. Because of that we are always
exposed to Radium and to small amounts of radiation that it
releases into the environment. Environmental effects of RadiumRadium is constantly produced by the
radioactive decay of Uranium and Thorium. Radium is present at
very low levels in rocks and soil and strongly attaches to those
materials. It is also found in air. High concentrations of Radium
exist in water on some locations. |