|
|
|
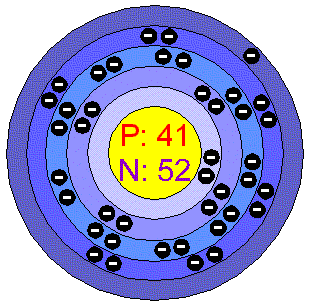
Niobium is a rare, soft, malleable, ductile, gray-white metal. It has a body-centered cubic crystalline structure and in its physical and chemical properties it resembles Tantalum. It must be placed in a protective atmosphere when processed at even moderate temperatures because it tends to react with Oxygen, Carbon, the halogens, Nitrogen, and Sulfur. The metal is inert to acids, even to aqua regia at room temperatures, but is attacked by hot, concentrated acids, and expecially by alkalis and oxidizing agents. Applications Niobium is used for the production of high-temperature-resistant alloys and special stainless steels. Small amounts of Niobium impart greater strenght to other metals, especially those that are exposed to low temperatures. Niobium carbide is used in cutting tools. It is used in stainless steel alloys for nuclear reactors, jets, missiles, cutting tools, pipelines, super magnets and welding rods. Niobium-tin and Niobium-Titanium alloys are used as wires for superconducting magnets capable of producing exceedingly strong magnetic fields. Niobium is also used its pure form to make superconducting accelerating structures for particle accelerators. Niobium alloys are used in surgical implants because they do not react with human tissue. Niobium in the envIronment Plants generally show only traces of Niobium and many have none at all, although some mosses and lichens can contain 0.45 ppm. However, plant growing near Niobium deposits can accumulate the metal to levels above 1 ppm. Niobium was mined chifely as columbite, and is formerly known as colombium (Cb). Another mined metal is pyrochlore and this is now the most important. The main mining areas are Brazil, which produce more than 85% on the world's Niobium, Zaire, Russia, Nigeria and Canada. World production is around 25.000 tonnes per year. The amount of unmined reserves is not known, but there are extensive deposits of pytochlore.
|