|
 |
|
 |
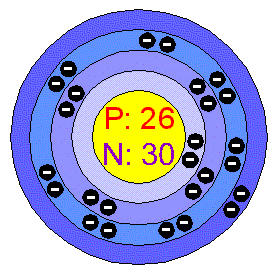
IronIron is a lustrous, ductile, malleable, Silver-gray metal (group VIII of the periodic table). It is known to exist in four distinct crystalline forms. Iron rusts in dump air, but not in dry air. It dissolves readily in dilute acids. Iron is chemically active and forms two major series of chemical compounds, the bivalent Iron (II), or ferrous, compounds and the trivalent Iron (III), or ferric, compounds. Applications Iron is the most used of all the metals, including 95 % of all the
metal tonnage produced worldwide. Thanks to the combination of low cost and high
strength it is indispensable. Its applications go from food containers
to family cars, from scredrivers to washing machines, from cargo ships
to paper staples. Iron in the environment Iron is believed to be the
tenth most abundant element in the universe. Iron is also the most
abundant (by mass, 34.6%) element making up the Earth; the concentration
of Iron in the various layers of the Earth ranges from high at the inner
core to about 5% in the outer crust. Most of this Iron is found in
various Iron oxides, such as the minerals hematite, magnetite, and
taconite. The earth's core is believed to consist largely of a metallic
Iron-Nickel alloy. Health effects of IronIron can be found in meat, whole meal products, potatoes and vegetables. The human body absorbs Iron in animal products faster than Iron in plant products. Iron is an essential part of hemoglobin; the red colouring agent of the blood that transports Oxygen through our bodies. Iron may cause conjunctivitis, choroiditis, and retinitis if it contacts and remains in the tissues. Chronic inhalation of excessive concentrations of Iron oxide fumes or dusts may result in development of a benign pneumoconiosis, called siderosis, which is observable as an x-ray change. No physical impairment of lung function has been associated with siderosis. Inhalation of excessive concentrations of Iron oxide may enhance the risk of lung cancer development in workers exposed to pulmonary carcinogens. LD50 (oral, rat) =30 gm/kg. (LD50: Lethal dose 50. Single dose of a substance that causes the death of 50% of an animal population from exposure to the substance by any route other than inhalation. Usually expressed as milligrams or grams of material per kilogram of animal weight (mg/kg or g/kg).) A more common problem for humans is Iron deficency, which leads to anaemia. A man needs an average daily intake pf 7 mg of Iron and a woman 11 mg; a normal diet will generally provided all that is needed.
|